About CellTrypase
How CellTrypase works
CellTrypase is a recombinant, animal origin-free trypsin-like enzyme derived from Fusarium oxysporum.
Similar to porcine trypsin, CellTrypase is a serine protease that cleaves peptide bonds specifically at the carboxyl side of lysine and arginine residues. Produced under controlled, consistent conditions and formulated as a highly purified fungal enzyme, CellTrypase is inherently less reactive and so more gentle on cells.
As a result, CellTrypase:
- Supports detachment of adherent cells while preserving membrane integrity and cellular function
- Requires no inactivation: simple dilution in buffer or medium is sufficient to quench enzymatic activity
- Is suitable for storage at 2–8 °C and ambient shipment, simplifying handling, storage and logistics
In-house GMP Manufacturing
CellTrypase is produced in-house via microbial fermentation using Bacillus sp., a gram-positive, endotoxin-free production strain. The process follows a quality management system compliant with EXCiPACT® GMP standards, ensuring full traceability and adherence to GMP/GDP requirements. All steps are carried out in a controlled environment with fully documented processes on animal origin-free equipment. The enzyme is manufactured without antibiotics, animal-derived materials, or substances with TSE/BSE risk.
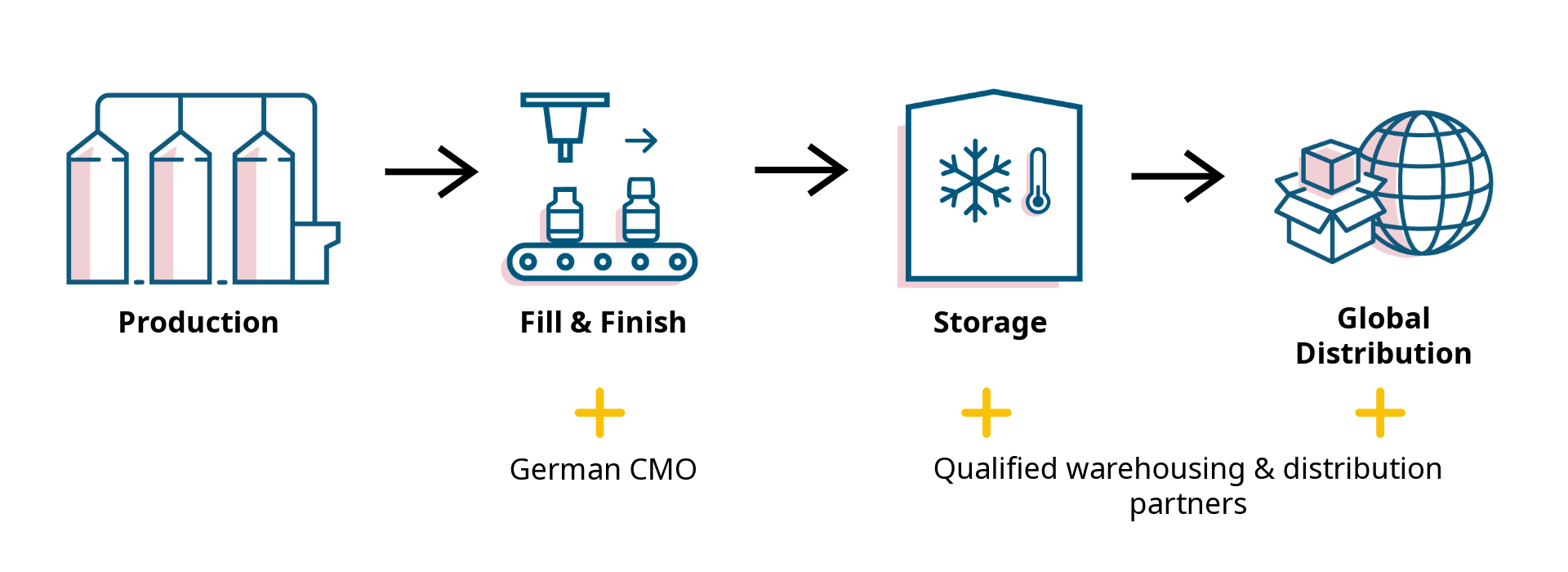
Reliable Supply & full Traceability
CellTrypase is supported by a fully controlled supply chain, managed by c-LEcta from fermentation to global distribution. Every step follows GMP/GDP standards to ensure quality and compliance.
We work with qualified partners for fill & finish, warehousing, and distribution to maintain the highest standards. This setup guarantees:
Purity you can see!
Our proprietary manufacturing process focuses on achieving a highly pure product and avoids steps such as spray drying, that may affect the integrity of the enzyme. The sharp HPLC peak of CellTrypase confirms ≥ 95% purity and the absence of major degradation products – in contrast to common industry trypsin solutions.
This high purity supports specific activity and consistent performance in sensitive cell culture workflows. A purity of ≥ 95% was therefore defined as a release-relevant parameter in the product specification, underscoring the stringent quality standards applied to CellTrypase.
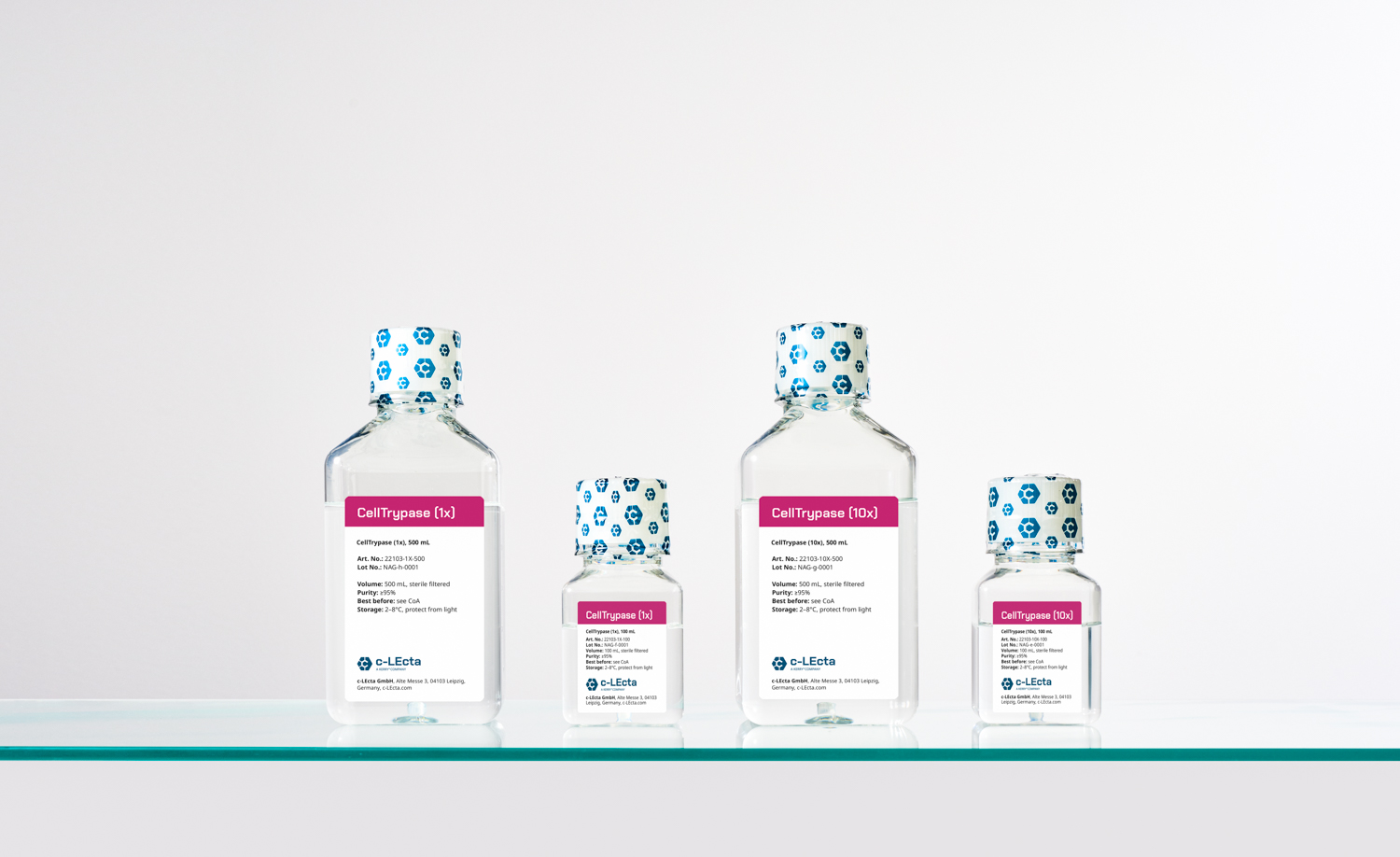

CellTrypase is available in two Quality Grades:
CellTrypase for Research and Development (R&D) use &
CellTrypase for manufacturing under GMP.
-
Both grades are produced using the same proprietary process in Bacillus sp., on animal origin-free equipment, and are free from antibiotics, animal-derived materials, and TSE/BSE risks.
-
CellTrypase GMP-grade is manufactured under a certified quality management system in line with the EXCiPACT® Certification Standard, meeting GMP/GDP requirements for pharmaceutical excipients. Full regulatory documentation is available on request to support product filings and submissions.
-
CellTrypase R&D-grade is produced under ISO 9001 standards.
-
Despite differences in documentation and compliance, both grades offer identical technical performance and meet the same product specifications, enabling a smooth transition from early R&D to GMP manufacturing.
Ready to switch?
CellTrypase integrates seamlessly into existing workflows and offers a reliable, animal origin-free alternative to conventional trypsin solutions – ideal for research, process development, and GMP-compliant manufacturing.

Order CellTrypase now!
Step 1 | Which product?
To match your workflow needs, CellTrypase is available in two concentration formats. Select the format that best fits your cell culture process.
Ready-to-use solution: No dilution needed, ideal for direct application.
10x concentrated stock: Flexible use. Dilute to 1x or apply undiluted for faster, gentle dissociation of tightly adherent cells.
Step 2 | Which quality grade?
CellTrypase is available in two quality grades to support your entire cell culture workflows from early-stage research to GMP-compliant manufacturing.
For research and process development using attachment-dependent cells. The enzyme enables gentle, reproducible dissociation while preserving cell viability and function. Ideal for adherent cell lines, stem and primary cells.
Purity: ≥ 95% (determined by HPLC)
Formulation: Liquid in PBS with EDTA, sterile filtered
Appearance: Clear to slightly colored solution
Storage: 2–8 °C
Delivery: 1–2 weeks (depending on region)
- In-house ISO 9001-compliant manufacturing
- Free from animal-derived raw materials and antibiotics
- Produced with animal origin-free machinery
- Net price excluding VAT and shipment costs
For GMP manufacturing using attachment-dependent cells. The enzyme enables gentle, reproducible dissociation while preserving cell viability and function. Ideal for key biopharma production cell lines used in viral vector and vaccine manufacturing, as well as for cell therapies using stem and primary cells.
Purity: ≥ 95% (determined by HPLC)
Formulation: Liquid in PBS with EDTA, sterile filtered
Appearance: Clear to slightly colored solution
Storage: 2–8 °C
Delivery: 1–2 weeks (depending on region)
-
In-house GMP production (QMS in compliance with EXCiPACT® certification standard)
-
Free from animal-derived raw materials and antibiotics
-
Produced with animal origin-free machinery
-
Dedicated regulatory support for pharmaceutical product approvals
-
Net price excluding VAT and shipment costs
Step 2 | Which quality grade?
CellTrypase is available in two quality grades to support your entire cell culture workflows from early-stage research to GMP-compliant manufacturing.
For research and process development using attachment-dependent cells. The enzyme enables gentle, reproducible dissociation while preserving cell viability and function. Ideal for adherent cell lines, stem and primary cells.
Purity: ≥ 95% (determined by HPLC)
Formulation: Liquid in PBS with EDTA
Appearance: Clear to slightly colored solution
Storage: 2–8 °C
Delivery: 1–2 weeks (depending on region)
-
In-house ISO 9001-compliant manufacturing
-
Free from animal-derived raw materials and antibiotics
-
Produced with animal origin-free machinery
-
Net price excluding VAT and shipment costs
For GMP manufacturing using attachment-dependent cells. The enzyme enables gentle, reproducible dissociation while preserving cell viability and function. Ideal for key biopharma production cell lines used in viral vector and vaccine manufacturing, as well as for cell therapies using stem and primary cells.
Purity: ≥ 95% (determined by HPLC)
Formulation: Liquid in PBS with EDTA
Appearance: Clear to slightly colored solution
Storage: 2–8 °C
Delivery: 1–2 weeks (depending on region)
-
In-house GMP production (QMS in compliance with EXCiPACT® certification standard)
-
Free from animal-derived raw materials and antibiotics
-
Produced with animal-origin-free machinery
-
Dedicated regulatory support for pharmaceutical product approvals
-
Net price excluding VAT and shipment costs
Get in touch with our CellTrypase expert team!

Vaishnavi Devarakonda
CellTrypase Sales

Melissa Rangel
CellTrypase Sales
Need support? Contact me!

Sedef Özyürek-Heistermann
CellTrypase Sales
Need support? Contact me!